Lesson Plan Overview
Students will use the atomic mass of an element often throughout any chemistry course. One common misconception is that some atoms have a mass equal to the atomic mass, but that is false in many cases. Because the atomic mass is a weighted average that reflects the abundance of isotopes in nature, a model helps students better understand this concept.
In this activity, students will create examples to illustrate the percent abundances for an element and calculate the atomic mass for an element. This will help students understand this idea visually. The example above can also be edited and used as an example for students rather than an activity. Copying the assignment will also copy the completed example into your account!
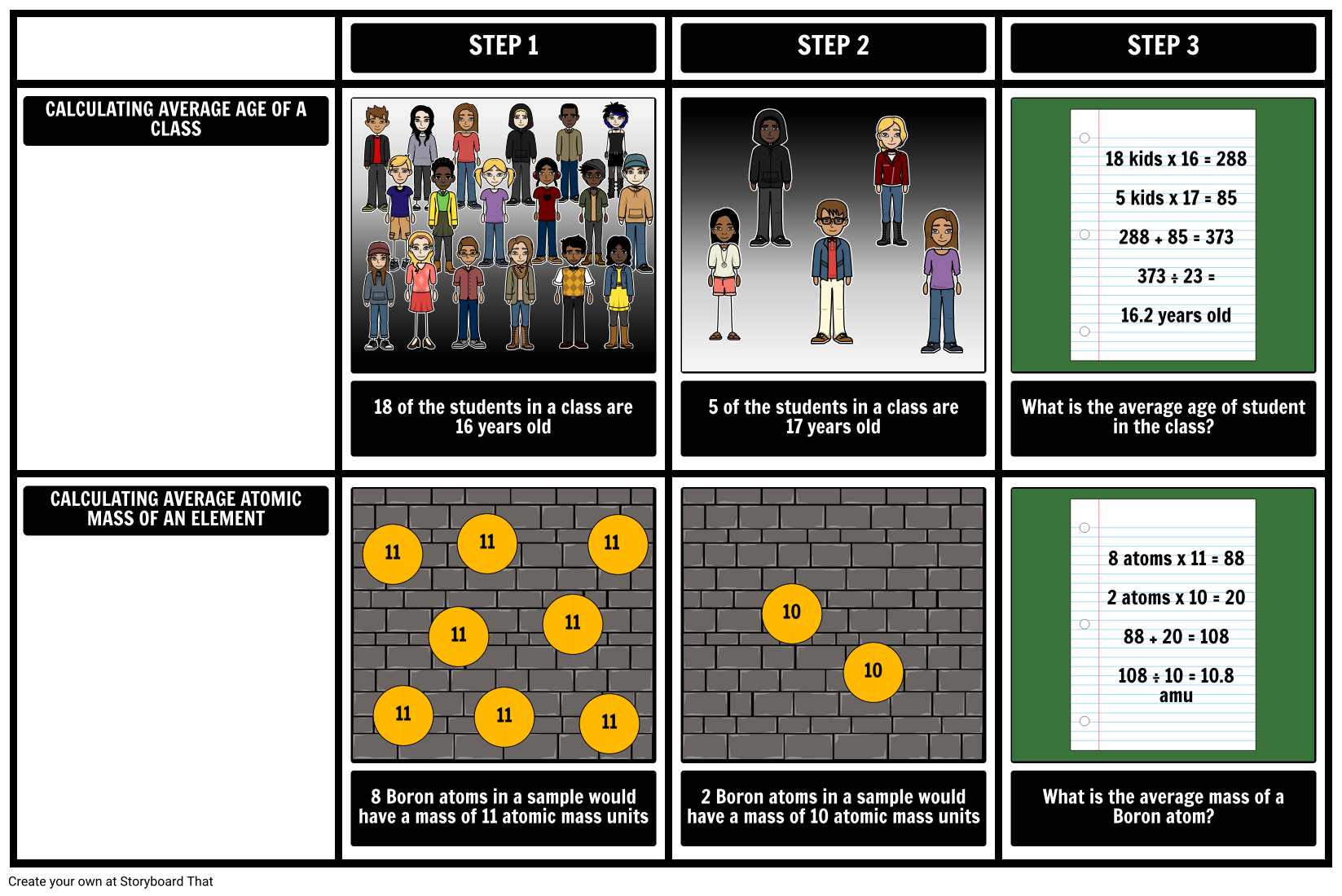
In order to calculate the atomic mass of an element, scientists must know what isotopes exist and how abundant they are. Once this is known, the average atomic mass is calculated by taking into account the masses of the isotopes and how prevalent they are. This can be compared to finding the average age of students in a class. When most of the students are 16, but some of the students are 17, the average age can be predicted to be close to 16. Students typically understand this intuitively. The average age is not necessarily 16.5 (the average of 16 and 17) unless their are equal numbers of students who are 16 or 17. This model can be extended to isotopes: the more abundant an isotope, the closer the average will be to the mass of that isotope and the average will not necessarily be the midpoint between the masses of the isotopes.
Template and Class Instructions
(These instructions are completely customizable. After clicking "Copy Activity", update the instructions on the Edit Tab of the assignment.)
Student Instructions
Create a chart that illustrates how to find the atomic mass of elements. Remember, the atomic mass is a weighted average.
- Click "Start Assignment".
- In the top row, calculate the average age of students in a class.
- In the bottom row, do the same for the average atomic mass for an element.
- Create an illustration to help visualize your calculations.
- Save and exit when you're done.
Lesson Plan Reference
Lesson Plan Overview
Students will use the atomic mass of an element often throughout any chemistry course. One common misconception is that some atoms have a mass equal to the atomic mass, but that is false in many cases. Because the atomic mass is a weighted average that reflects the abundance of isotopes in nature, a model helps students better understand this concept.
In this activity, students will create examples to illustrate the percent abundances for an element and calculate the atomic mass for an element. This will help students understand this idea visually. The example above can also be edited and used as an example for students rather than an activity. Copying the assignment will also copy the completed example into your account!
In order to calculate the atomic mass of an element, scientists must know what isotopes exist and how abundant they are. Once this is known, the average atomic mass is calculated by taking into account the masses of the isotopes and how prevalent they are. This can be compared to finding the average age of students in a class. When most of the students are 16, but some of the students are 17, the average age can be predicted to be close to 16. Students typically understand this intuitively. The average age is not necessarily 16.5 (the average of 16 and 17) unless their are equal numbers of students who are 16 or 17. This model can be extended to isotopes: the more abundant an isotope, the closer the average will be to the mass of that isotope and the average will not necessarily be the midpoint between the masses of the isotopes.
Template and Class Instructions
(These instructions are completely customizable. After clicking "Copy Activity", update the instructions on the Edit Tab of the assignment.)
Student Instructions
Create a chart that illustrates how to find the atomic mass of elements. Remember, the atomic mass is a weighted average.
- Click "Start Assignment".
- In the top row, calculate the average age of students in a class.
- In the bottom row, do the same for the average atomic mass for an element.
- Create an illustration to help visualize your calculations.
- Save and exit when you're done.
Lesson Plan Reference
How Tos about Atomic Mass of Elements: Calculation Activity
How to Introduce Isotopes and Atomic Mass with Everyday Objects
Engage students by using familiar, hands-on materials to model isotopes and weighted averages, making abstract chemistry concepts more accessible.
Gather common classroom items for modeling
Collect objects such as different colored candies, coins, or paper clips to represent various isotopes. Choose items that are easy to sort and count so students can focus on the activity.
Assign masses and abundances to each object type
Label each object type with a mass (e.g., red candy = 10 units, green candy = 12 units). Decide on the number of each type to represent isotope abundance (e.g., 8 red, 2 green for 80% and 20%).
Guide students in calculating the weighted average
Instruct students to multiply the mass of each object type by its fraction of total objects, then add the values together. Explain how this models calculating atomic mass from isotopes.
Discuss real-world connections and misconceptions
Facilitate a conversation about how their model relates to real elements and why atomic mass isn’t always a whole number. Address common misunderstandings and link back to periodic table values.
Frequently Asked Questions about Atomic Mass of Elements: Calculation Activity
What is atomic mass and how is it calculated for elements?
Atomic mass is the weighted average mass of all naturally occurring isotopes of an element. It is calculated by multiplying the mass of each isotope by its natural abundance, then summing these values. This reflects both the isotope's mass and how common it is in nature.
Why isn't the atomic mass always a whole number?
Atomic mass isn't always a whole number because it is a weighted average based on the different isotopes of an element and their natural abundances. Most elements have multiple isotopes with varying masses, so the average falls between whole numbers.
How can I help students visualize atomic mass using classroom activities?
You can help students visualize atomic mass by creating a chart or hands-on model that compares calculating the average age of students in a class to finding the average atomic mass of an element. This makes the concept more relatable and concrete.
What's the difference between atomic mass and mass number?
Atomic mass is the weighted average of all isotopes' masses for an element, while the mass number is the total number of protons and neutrons in a specific atom's nucleus. Atomic mass considers isotope abundance; mass number is for a single atom.
What is a simple classroom activity to teach atomic mass calculation?
A simple activity is to have students create a chart comparing the average age of students (using class data) to the average atomic mass of an element (using isotope data). Adding a visual illustration helps students understand weighted averages in both cases.
More Storyboard That Activities
Understanding Atomic Structures
Testimonials

“By using the product, they were so excited and they learned so much...”–K-5 Librarian and Instructinal Technology Teacher

“I'm doing a Napoleon timeline and I'm having [students] determine whether or not Napoleon was a good guy or a bad guy or somewhere in between.”–History and Special Ed Teacher

“Students get to be creative with Storyboard That and there's so many visuals for them to pick from... It makes it really accessible for all students in the class.”–Third Grade Teacher
© 2026 - Clever Prototypes, LLC - All rights reserved.
StoryboardThat is a trademark of Clever Prototypes, LLC, and Registered in U.S. Patent and Trademark Office